B2 Formulation and stabilisation of the obtained mixtures
The main objective of this action was the formulation of three high quality, environmentally friendly products suitable for zero waste agriculture, based on bioactive extracts obtained on a pilot scale. The formulations had to meet standards for storage, transport and safety conditions. They also had to be tested for industrial scale-up and possible harmful effects on plants had to be ruled out to confirm their commercial viability. Finally, a basic analysis of their potential registration pathway in the European Union was required.
Conclusions
- The study, search and choice of co-formulant raw materials was successful in obtaining suitable formulas containing the selected extracts. In addition, a low-risk toxicological profile was sought for each of the raw materials, compatible with their certification in Organic Agriculture. With regard to the ecotoxicity evaluation of the formulated matrices, the results obtained were acceptable for the intended use.
- The numerous formulation and characterisation tests carried out led to the selection of 3 prototypes. These formulations met the internal standards and were used for further scale-up. Physicochemical analyses were carried out on these 3 final formulations for the elaboration of their technical data sheet (FTP) and safety data sheet (SDS).
- These three prototypes were scaled up in two stages to a mixing volume of 500L and the process was validated. The formulas obtained were tested in the field to assess their phytotoxicity, showing no damage to the crop under both greenhouse and open field conditions.
- Finally, a preliminary analysis was carried out on the possibility of the final formulations to be registered under the classification of Basic Substance within the Regulation 1107/2009 for the use of plant protection products in agriculture.
B.2.1.- Planning, sourcing and procurement of raw materials
Within this task, suitable raw materials were evaluated and selected for use in the development of formulations based on the candidate plant extracts.
Conclusions
Firstly, a literature search was carried out to find acceptable substances compatible with the extracts, including: potential solvents, adjuvants with the ability to homogenise complex mixtures, emulsifiers to enable the final product to be sprayed, and physical/chemical stabilisers. Their commercial availability, their positive compatibility with organic certification, their toxicological profile (CLP hazard) were then assessed and samples of these substances were collected for subsequent use in formulation feasibility tests.
B.2.2.- Planning and execution of test batteries
The objective of this action was to design and execute a test battery for the selection of a small number of viable prototypes for subsequent scaling and testing of different properties.
Conclusions
The extracts were integrated in different formulation matrices, specially designed for this project, with a variable content of solvent, adjuvants and different enhancers.
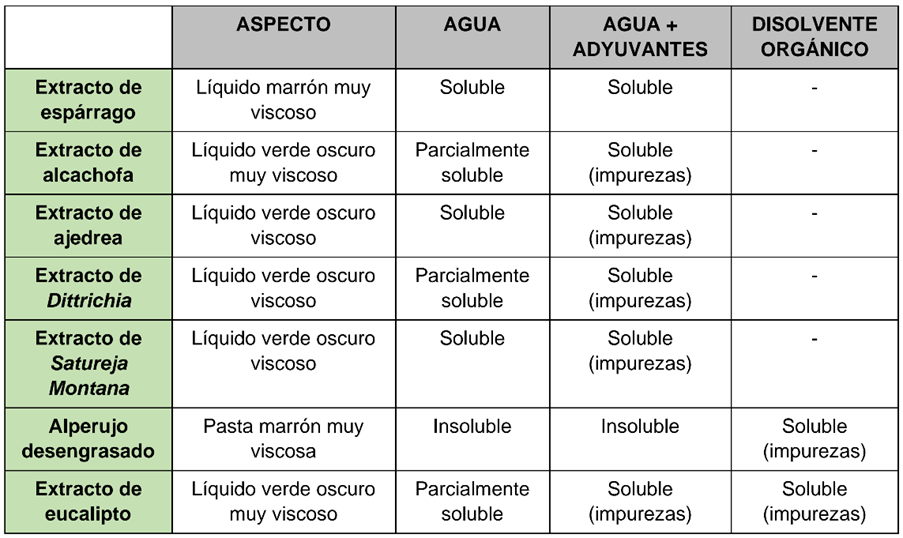
Summary table of some physico-chemical properties obtained for the candidate extracts
Observing the results according to the table above, more than 40 different tests were designed to obtain a suitable matrix for each extract. At the end of each test, parameters such as pH, density and solubility, among others, were analysed in triplicate. The presence of pesticide residues, heavy metals and prohibited substances was analysed for each of the formulations by external accredited laboratories. Final physicochemical analyses were carried out, and these were used to draw up the corresponding technical and safety data sheets.
Combinations that did not meet characteristics such as extreme pH, precipitates, phase differences, crystallisation, vapour release or presence of prohibited substances were discarded. For each extract, those formulations were selected that were homogeneous and stable for at least 48 hours at three temperatures: 3, 25 and 45 degrees Celsius.
In this way, 4 matrices compatible with the extracts were obtained, which were tested and gave rise to 9 formulation prototypes. The same battery of analyses was carried out on them as described above for the selection of matrices.

Detail of some of the prototypes obtained
In parallel, the ecotoxicological evaluation of the 4 selected matrices was carried out directly on the real materials on an experimental basis at the Instituto de Salud Carlos III. These studies gave rise to the results summarised in the following table:
Summary table of ecotoxicological data obtained for the 4 selected matrices
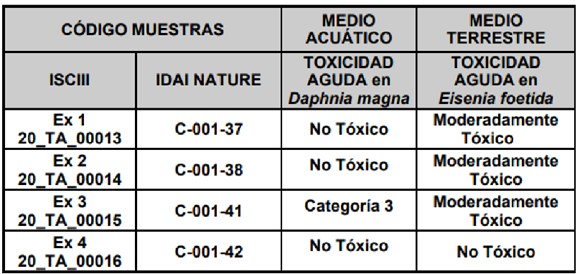
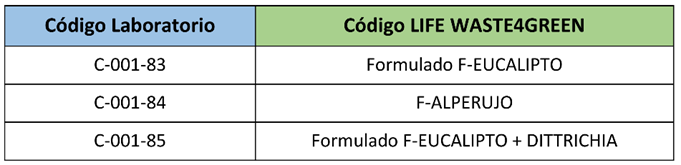
After sharing all the data obtained for formulation, toxicity and in vitro effectiveness with the other participants (CSIC, ISCIII and CTAEX), it was decided that the extracts selected to continue with the field trials would be the Alperujo Extract, the Eucalyptus Extract and the Eucalyptus-Dittrichia synergic extract.
List of the 3 prototypes that underwent further optimisation and validation tasks
B.2.3.- Formulation optimisation
In order to carry out the scaling of the selected prototypes to validate their viability for industrial manufacture.
Conclusions
Several batches of each formulation were prepared in IDAI's pilot reactors, with a capacity of 5L and 50L, to validate the stages of the process developed in the laboratory. In this scale-up stage, an attempt was made to faithfully reproduce the laboratory conditions, previously established in task B2.2. In most of the formulations, variations/adaptations were made with respect to the laboratory scale process to adjust the steps to the conditions of the semi-industrial reactors, as part of the optimisation of the formulations.

Detail of the 5-litre and 50-litre reactors used in the scaling process.
Once this point was reached, the optimised process was adapted to the next scale. In this case, an industrial reactor with a capacity of 1000L, where different batches of each prototype were formulated to repeat the process. This allowed validation of the product obtained with respect to the parameters established during the laboratory development.

Reactor de 1000L utilizado en el proceso de escalado
The objective was successfully achieved and allowed the preparation of the necessary material for the needs of the project according to the availability of extracts.
B.2.4.- Pre-commercial validation of the products
A test was carried out to evaluate the phytotoxicity of the 3 selected prototypes on model crops.
Conclusions
The trial was conducted under both greenhouse and open field conditions. In terms of design, 21 plants of each species [Chard (Beta vulgaris) and Romanesco (Brassica oleracea)] with similar appearance and development were selected. These were divided into groups for each formulation, which was tested at two concentrations (5 ml or 10 ml); each group included 3 plants and there were 3 replicates of each treatment. Only one foliar application was made. Possible injuries or visible negative effects on the plants were evaluated.


No differences are observed between the control thesis (left) and the treatment (right). Example in potted plants (greenhouse)
No symptoms of phytotoxicity were observed at 24h, 72h and 7 days in any of the two crops and in any of the applications, regardless of whether they were tested in the greenhouse or in the field.
B.2.5.- Registration of new products as Basic Substances
The aim of this action is to study the possibility that the solutions developed in LIFE Waste4Green, specifically the Eucalyptus and Alperujo extract, can be registered under the classification of Basic Substance within Regulation 1107/2009 for the use of plant protection products in agriculture.
Conclusions
During the development of the project, Eucalyptus and Alperujo were selected as substances that met the criteria of the research project in terms of biomass availability, higher in vitro biological activity, lower risk to human health and the environment, as well as their capacity to be formulated as marketable products in agriculture.
These criteria have served to generate very useful knowledge about the use of waste wood pulp and eucalyptus from the circular economy for pest and disease control in agriculture. However, the documentation generated is not sufficient for the submission of these as candidate basic substances under Regulation (EC) 1107/2009.